Sugar Wash pH Control Experiment #1

- The first batch is controlled with a pH buffer we made from citric acid and sodium hydroxide.
- The second batch we did a ‘control batch’ where we didn’t add any pH controllers until halfway through.
- For the third batch, we prepared live oyster shells and put them in the fermenter.
- The fourth batch is adjusted with baking soda as required.
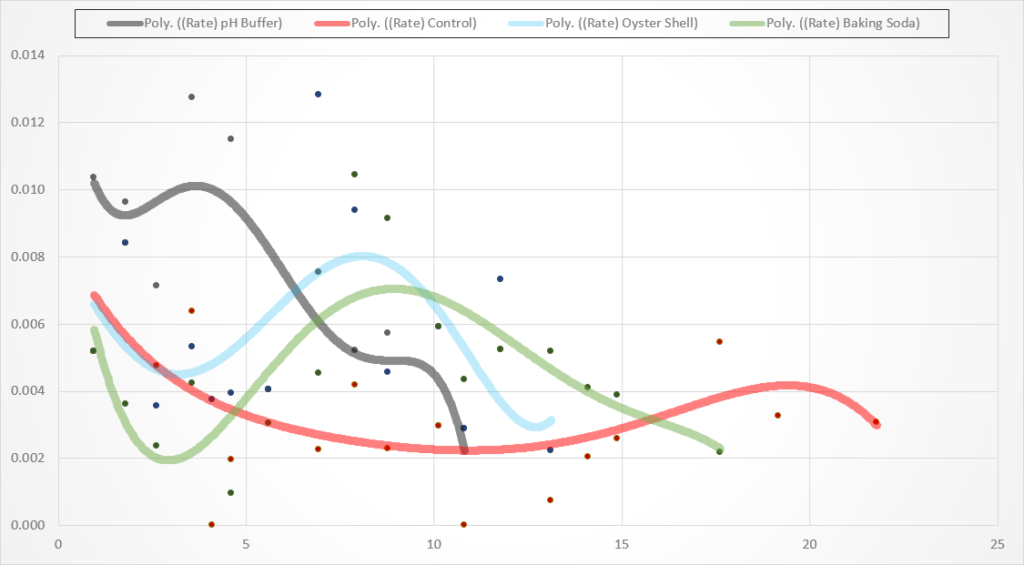
Below are the scatter charts with trend lines fitted to the data. Each line ends when the batch hits a specific gravity of 0.992. Keep in mind that the red “control” batch actually had 2 full oyster shells added around day 13 as it had a sluggish fermentation and I lost my patience with waiting around for it to ferment. I also added additional oyster shells to batch 3 (the oyster shell batch) at day 4. See the notes section below for more detail.
I’ve included a pic of the oyster shells after fermentation. They were very white, where most of the black outer shell coloring melted off during fermentation. They were also very brittle but eventually hardened after drying out. They were much thinner after being removed and definitely dissolved. I was able to crack the shells with my fingers no problem. I’ll reuse what’s left in future experiments.

Graphs


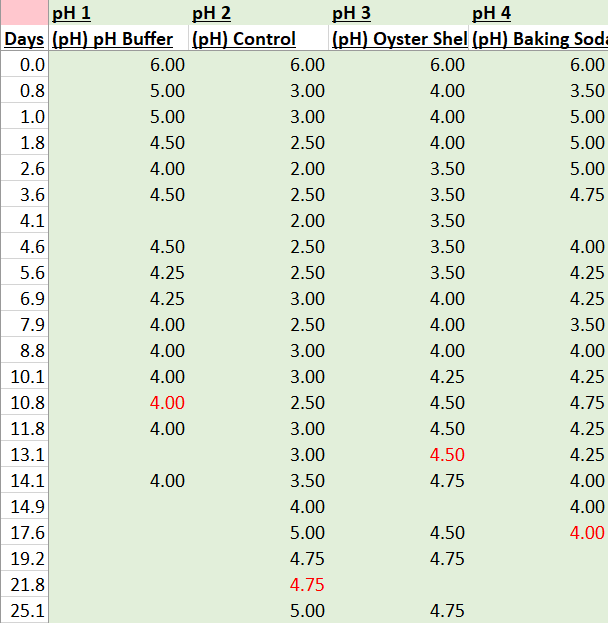
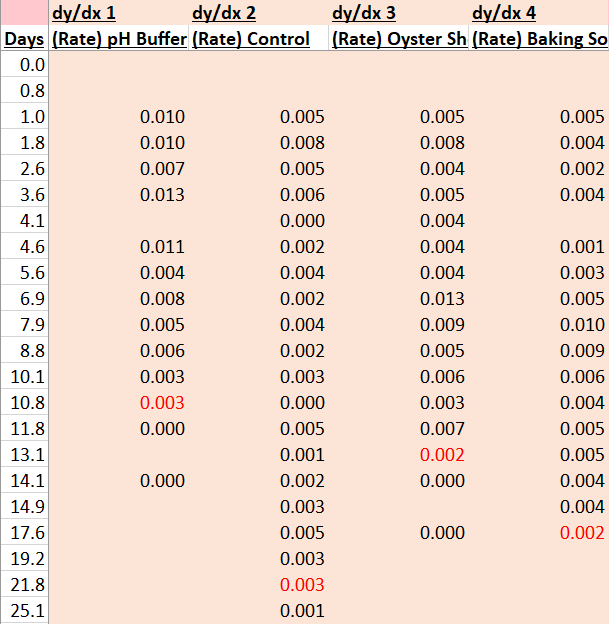
Raw Data
Here is the raw data from the experiments. I have highlighted values in red that correspond to when each batch reached a specific gravity of 0.992 (what I am considering terminal gravity for this experiment).





2 Comments
Ralph · November 24, 2019 at 7:49 AM
Do you know how much sodium hydroxide you can add before the sodium starts to impact the yeast? I started using sodium bicarbonate to reduce sugar wash acidity, but recently switched to calcium hydroxide.
Joey Joe Joe Jr · November 24, 2019 at 11:32 AM
I haven’t done any experiment around that. I did, however, find a study that did an experiment with NaCl and wine yeast. They are using weight volume numbers in this study, meaning what percent of the water is NaCl by weight, so a 20 liter 1% solution of NaCl would have 200 grams of NaCl in it. Looks like at 1% there are negative effects, and they get worse with more NaCl. I assume the same would be true with other chemicals adding sodium ions. Cheers and thanks for watching. https://www.dovepress.com/influence-of-sodium-chloride-on-wine-yeast-fermentation-performance-peer-reviewed-article-IJWR